Quantifying membrane dynamics to gain insights into cell function
Narain Karedla and colleagues at The University of Göttingen have developed a method to capture membrane fluctuations with unmatched spatio-temporal resolution. The versatility of metal- and graphene-induced energy transfer spectroscopy is shedding new insights into how membrane composition influences its mechanical properties.
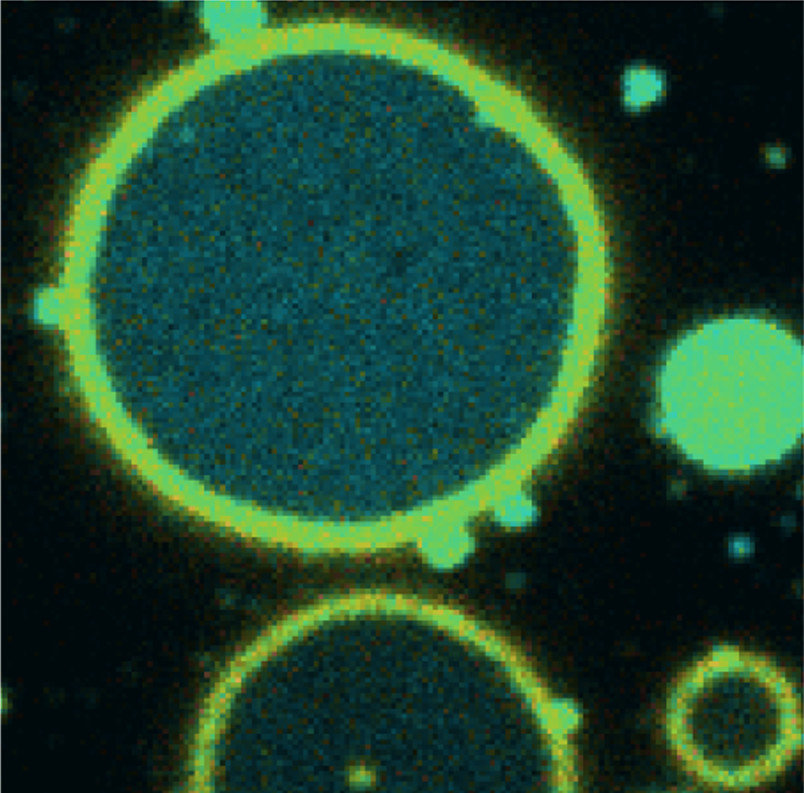
Biological membranes that surround cells and organelles consist of various lipids and transmembrane or peripheral proteins that form a selectively permeable barrier between cellular compartments. Far from being static, they behave as two-dimensional fluids — lipids and proteins can rotate and move laterally within the bilayer — and they exhibit shape fluctuations, also known as undulations.
Although the importance of cell membrane fluctuations for cell adhesion, migration and communication has been recognised for decades, few researchers are studying them. This is due, at least in part, to the technical challenges associated with accurately measuring undulations taking place at nanometre (nm) length scales and sub-millisecond timescales.
“Membranes behave like water-filled balloons,” says Narain Karedla, advanced microscopy specialist at the Rosalind Franklin Institute. “The fluctuations reflect the mechanical properties of the membrane, which are determined by their composition, as well as physical processes that are taking place, such as the actin cytoskeleton pushing against membranes and ion channel pumping.”
The best characterised membrane fluctuations are those of red blood cells (RBCs), which can be observed to ‘flicker’ using ordinary light microscopy. “RBCs exhibit significant fluctuations that allow them to move around the body and prevent them from sticking to blood vessel walls,” Karedla explains.
Using interference (phase contrast) microscopy, Brochard and Lennon were the first to quantify RBC membrane fluctuations in 1975. Since then, these fluctuations have been extensively characterised using various methods including fluorescence interference contrast and reflection interference contrast microscopy, which measure intensity variations over time to calculate the height fluctuations of the membrane surface.
However, as Karedla points out, while membrane fluctuations that are between 50-100 nm in height are easily captured by intensity interference-based methods, they can’t detect ones produced by membranes with large surface tension that are in the order of 10 nm or less.
To overcome this problem, he has been working with Jörg Enderlein and Tao Chen at the Georg August University of Göttingen in Germany, to develop a metal-/graphene-induced energy transfer (MIET/GIET)-based method whereby the distance of a fluorescent dye to the metal/graphene surface changes the fluorescence lifetime of the dye. The change in the fluorescence properties of the dye labelling the membrane, due to the distance-dependent energy transfer from the dye to the surface of a metal or a single sheet of graphene, allows the researchers to establish the height of the membrane.
“Our theoretical model calculates the change in fluorescence lifetime of the dye as a function its distance from the surface with nanometer accuracy,” Karedla explains.
“Our theoretical model calculates the change in fluorescence lifetime of the dye as a function its distance from the surface with nanometer accuracy,”
In a paper published in Nature Communications, they show that both fluorescence lifetime and intensity variations correlate with membrane height changes1. “By combining MIET/GIET with fluorescence correlation microscopy (FCS), we can measure tiny 3 nm oscillations of a fluorescently-labelled stretched membrane with microsecond time-resolution,” he says.
Karedla and colleagues tested the optical properties of many materials. With gold-covered cover slips they found they could clearly measure undulations over 10-15 nm in height, but with graphene the resolution improved by an order of magnitude.
When they applied MIET-FCS to RBCs, they confirmed previous findings showing that membrane fluctuations are higher in the presence of adenosine triphosphate (ATP), a nucleotide that powers many processes in living cells. To explore the membrane dynamics of membranes with a much larger surface tension, such as pore-spanning membranes and mitochondrial membranes, they used GIET-FCS.
Mitochondria, known as the powerhouses of cells, import adenosine diphosphate and export ATP. With GIET, the researchers were able to detect nanometer decreases in inner mitochondrial membrane height in the presence of ADP (active state), which facilitates the transport and exchange of molecules between the inner and outer membranes, compared with the resting state in the absence of ADP. “These measurements are indicative of mitochondrial health, which is affected in diseases such as non-alcoholic fatty liverdisease (NAFLD),” he says.
Karedla will continue to work with Enderlein to improve the resolution of methods to study membrane dynamics, and as aconfocal microscope with fluorescence lifetime imaging capability is in the process of being set up at the Institute, he is looking forward to collaborating with colleagues in Oxford and beyond.
“Several group leaders have already expressed an interest in applying MIET/GIET-FCS to look at the effects of lipid composition and charge on cell tension and adhesion properties in different immunology contexts,” he says. He is also excited about the possibilities of combining MIET/GIET with other techniques such as cryo-electron microscopy to examine the position and structural conformation of membrane proteins.
Karedla completed his PhD under the supervision of Enderlein in 2016. “Narain graduated as the top PhD student of the Faculty of Physics, an achievement that led to the publication of his thesis as a Springer monograph entitled ‘Single-Molecule Metal-Induced Energy Transfer: From Basics to Applications’,” Enderlein recalls. “Despite moving on from the University of Göttingen, we have maintained a close and highly productive collaboration; our ongoing partnership has resulted in over half a dozen significant publications, particularly focusing on MIET and its applications to various biophysical and life science challenges,” he adds.
Reference: Chen, T., Karedla, N., & Enderlein, J. (2024). Measuring sub-nanometer undulations at microsecond temporal resolution with metal- and graphene-induced energy transfer spectroscopy. Nature communications, 15(1), 1789. https://doi.org/10.1038/s41467-024-45822-x