The aim
Developing cutting edge techniques to image within the most native setting possible on the molecular scale so we can identify earliest structural changes leading to disease onset.
“We want to better understand how healthy cells function, and what goes wrong on the smallest scale to cause diseases such as Alzheimer’s or Parkinson’s. We will develop and work alongside new technologies to bring about new insights into the inner workings of diseases. By building a holistic picture of fundamental visual and genetic basis of structural cell biology within the disease context, we hope to help design better treatments for diseases in future ”

The ambition
Histological examination of samples is routine but can often lead to misdiagnosis and a lack of understanding of the underlying pathology. By linking pathology to molecular footprints, we hope to one day allow the earliest time points of disease to be understood to the point that we may help inform intervention based on a mechanistic understanding of disease.


What are we doing?
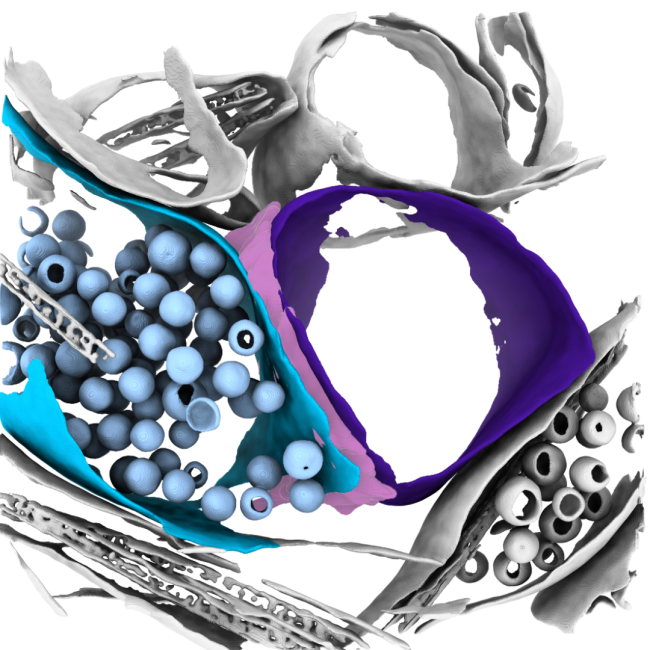
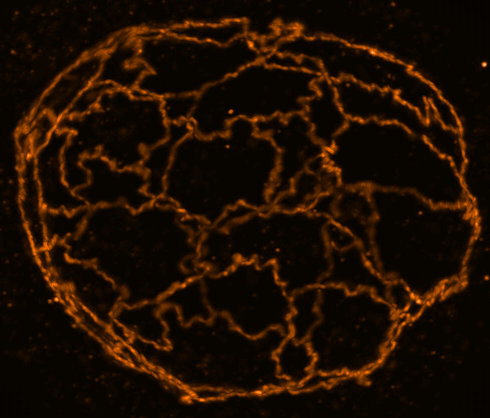
The goals of this research initially are to develop focused ion beam milling combined with electron cryo-tomography (cryoET) to unravel the molecular details of processes within cells and tissues, ultimately leading to an understanding of how changes in the structures of macromolecules in their native context impact on cellular function. Combining this approach with other techniques could scale insight into holistic human disease models spanning genes to function.
The formation of neuropathic phenotypes in-situ is poorly understood. We are developing approaches to investigate the morphological and structural changes that occur within tissues during the development and onset of disease using cryoET. In the example of neurodegenerative diseases, the formation of inclusion bodies and neurofibrillary tangles are well-known and prominent features of late-stage disease; however, we aim to understand the molecular changes that occur in proteins that give rise to these features at different stages of disease in-situ. We are developing approaches to image some of these molecular details in human patient derived samples, so that we can best understand what early disease looks like.
Why?
Errors in proteins lead to disease. It is important to study structures of these proteins in as close as possible to their native state and in contexts that can recreate potential disease states to best characterise how these errors impart dysfunction.
As an exemplar, techniques are being developed around a range of neurodegenerative questions, including proteins implicated in Alzheimer’s Disease and Parkinson’s Disease, utilising several model tissues systems from different organisms.
Why the Rosalind Franklin Institute?
The ability to utilise the cutting edge technologies at the Franklin, alongside working with experts at the Franklin and instrument manufacturers, is what makes this project possible.