New bifunctional, bispecific nanobodies scaffold helps with small protein imaging
Researchers at the Rosalind Franklin Institute, University of Oxford, and Diamond Light Source have combined their expertise to create new scaffolding molecules to allow the electron microscopy imaging of small proteins (below 50 kDa). Using this method the team have solved the smallest protein structure to date by cryoEM, that of hen egg white lysozyme, which is just 14 kDa.
The ability to image these small proteins is important, as proteins of this size range make up about 74% of protein-coding genes in humans, many of them playing important roles in how cells function and in disease.
In recent years, single-particle cryoEM has become a mainstream method for determining protein structures. One of the technique’s main advantages is the ability to show the molecular details of structures in a near native state.
Despite the growing popularity of cryoEM as a technique, it is still challenging to image small proteins using it. One of the major problems is the low signal-to-noise ratio of these small particles, which leads to difficulty during data processing with particle picking and alignment, and ultimately historically leading to low resolution reconstructions. The new method described overcomes these data processing challenges by bonding the small proteins to the ends of bifunctional, bispecific nanobodies, which increases apparent size and gives them a distinct geometry.
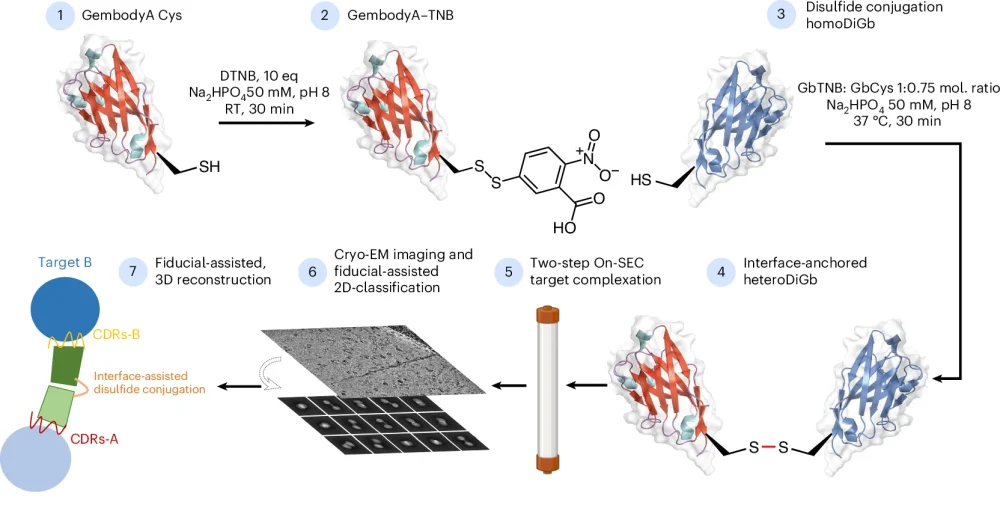
Dimitrios Mamalis, a joint PhD student between at the University of Oxford and the Franklin, explains how this work got started, “It started as a Friday afternoon experiment, which means that it wasn’t the main focus of our research at the time, but it has ended up in a great result.
“Mingda [Ye] was originally working on Gembodies for crystallography and then we wondered if we could transfer this method to cryoEM if we could conjugate the Gembodies in solution. This was when I started to find the method and optimise the reactions and conditions, linking up with Gangshun [Yi] to get the structures solved.”
“This idea was initiated when solving sub-50kDa protein structures by cryo-EM was almost impossible. To break this barrier, many world-class scientists in different fields have combined forces and it is a great honour to work with them to bring this game-changing tool into reality!” adds Mingda Ye, postdoctoral scientist at the University of Oxford.
One of the big advantages of this method over other similar methods is its modularity and that it does not require specific optimisation to attach different proteins.
Another advantage of the technique is that the complexes can be heterodimeric, meaning that the structure of two molecules can be determined at the same time. The two proteins attached to the scaffold can also be different sizes.
Professor Ben Davis, Science Director at the Franklin, and paper co-author said, “This was a wonderful, organic collaboration between our various groups that grew out an analogy between the kinetic control that crystallisation affords as a ‘trap’ of protein-protein interfaces and the potential to use kinetically-controlled covalent bond formation in solution in a parallel manner to chemically ‘trap’. As Dimitri notes, it was a wonderfully clear hypothesis that worked strikingly well – the efficiency for such a sidechain-to-sidechain conjugation is notable and highlights the bias that the interface has to assemble and so enable trapping. It seems that this could be a very pragmatic use of protein conjugation.”
