New scanning technique overcomes longstanding challenge in cryo-SEM imaging
Researchers at the Rosalind Franklin Institute have developed a new approach to scanning electron microscopy (SEM) that dramatically reduces obstructions caused by charging artefacts in frozen biological samples. The advance, described in a paper published in Nature Communications, allows scientists to capture clearer, more accurate images of cellular structures in their native state – opening up new possibilities for studying biological processes in health and disease. This approach also has the potential to be applied to non-biological materials such as semiconductors.
SEM is a powerful technique for visualising biological specimens at high resolution, especially when combined with focused ion beam (FIB) milling to build up 3D reconstructions of tissue. When performed under cryogenic conditions – known as cryo-SEM – this technique can reveal intricate biological detail without the need for chemical fixation or staining. But until now, there has been a catch.
“When you’re working with frozen biological samples, imperfections known as charging artefacts become a big problem,” says Dr Maud Dumoux, Deputy Challenge Lead at the Franklin. “These samples do not conduct electricity, so as the microscope’s electron beam scans the surface, electrons get trapped, causing dark streaks and prohibiting imaging of part or the entire sample.”
The issue is particularly pronounced in regions rich in fatty molecules called lipids, such as lipid droplets themselves or myelinated axons (nerve cells wrapped in a fatty layer) in brain tissue. These artefacts not only obscure biological structures but have often forced researchers to chemically alter their samples in order to reduce charging – compromising the native state that cryo-SEM aims to preserve.
The breakthrough came from a collaboration with Franklin Staff Scientist Dr Abner Velazco, a specialist in scanning transmission electron microscopy (STEM) who had been exploring ways to reduce beam damage in STEM by changing how the beam scans across the sample.
“Normally, the scan goes from left to right, and from top to bottom,” says Abner. “But we found that if we altered that pattern – jumping between positions rather than scanning sequentially – we could reduce the accumulation of secondary effects that cause damage. And charging artefacts in SEM seemed to behave in a very similar way to those we observed in STEM.”
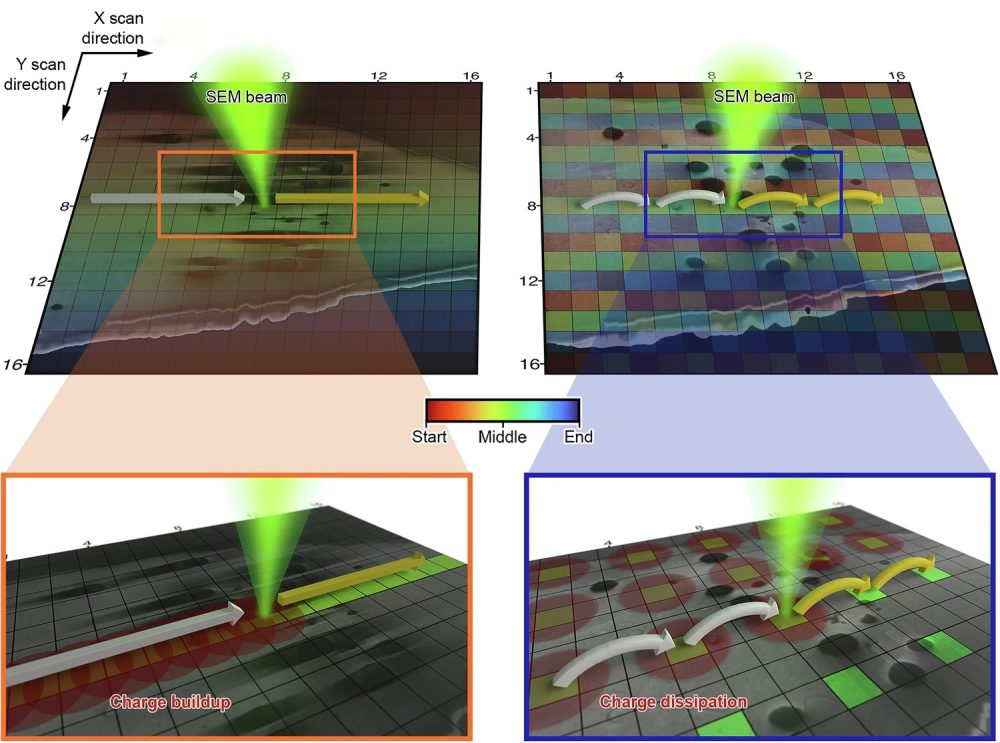
Recognising the parallels between beam damage and charging, the team decided to apply this alternative scanning method – known as interleaved or ‘leapfrog’ scanning – to cryo-SEM. Instead of scanning each pixel in order, the beam skips over areas in both the x and y directions, distributing the electron dose more evenly and allowing time for charge to dissipate.
With support from Quantum Detectors, the team integrated a customised scanning engine into their microscope setup to test the method. The results were immediate and striking.
“We went from images that were unusable due to distortion, to images where we could clearly see previously hidden structures. It has been a game-changer. People in the field have been really interested in this development”
The team demonstrated the method across a range of biological samples, including mammalian cells, algae, and mouse brain tissue. Notably, they were able to image the full structure of myelinated axons in an adult mouse cortex – something that had previously been avoided due to severe charging artefacts. “We’ve opened the door to imaging biological features that people thought were impossible to characterise with cryo-SEM,” adds Maud.
Equally important, the new method preserves the integrity of the biological sample. Maud says: “People used to change their cryo-SEM samples – embedding them in resin or bathing them in chemicals – to deal with artefacts. This technique allows the sample to remain as it is and lets us see the biology without interference.” The method could play a key role in human health research by enabling high-resolution, artefact-free imaging of unique patient samples and supporting detailed studies of tissue organisation in conditions such as ageing, infection and disease.
The team has made the acquisition code freely available on GitHub, allowing others in the field to adapt it to their own instruments. While the current method uses a specific scanning pattern, the researchers are already working on optimising it further.
“We’re now building mathematical models that simulate the charging process and scanning distortions,” adds Abner. “That should help us find the best possible scanning sequence for any given sample, and even correct for the small distortions that jumping between scanning positions introduces.”
The long-term vision is to integrate this method into a wider imaging pipeline. The team is also exploring how the same approach might be used during FIB milling itself, which can cause damage to samples during the preparation phase. And they’re developing user-friendly tools to help researchers automatically find the optimal scan settings for their own samples.
“This is just the beginning,” says Maud. “It’s a first step, but it’s already having a big impact. We want to keep pushing it, and make it available to the whole community.”

